Parkinson’s disease is horribly ubiquitous. The devastating neurodegenerative disease, which plays havoc with the central nervous system, affects up to 1% of people aged over 60. It can also hit younger people, though: famously, actor Michael J Fox was just 29 when he was diagnosed. Currently, there are about 10 million sufferers around the world – a number that researchers estimate will at least double by 2050, as global life expectancy continues to rise.
Unfortunately, there’s one thing everyone knows about Parkinson’s: it’s irreversible. Symptoms start with loss of motor control, such as tremors and instability, and eventually progress to a bewildering array of nasty manifestations that can include depression, mood swings, poor impulse control, loss of smell and even hallucinations and psychosis. While some medications can alleviate symptoms, they don’t stop the disease. Without intervention, patients will continue to suffer, and their decline will still be inevitable.
Moving beyond palliative treatments
Realising the need for disease-modifying therapies that can slow or halt neurodegenerative progression instead of just providing temporary relief, a CUHK research team has come up with a promising new gene therapy. This approach centres on a special peptide that’s injected directly into the brain, and it’s demonstrated some remarkable effects in experiments on mice. With just a single injection, it can slow the progression of the disease, reduce the amount of degeneration in the brain and provide protection against symptoms.

The team’s leader, Professor Michael Kenneth Chan from CUHK’s School of Life Sciences, says it’s previously been difficult to develop therapies for Parkinson’s disease because of the condition’s complicated and enigmatic nature.
“Parkinson’s disease is a complex neurodegenerative disorder with both genetic and environmental risk factors. While research has advanced our understanding, the exact cause remains unclear, making it challenging to develop targeted therapies that can halt disease progression. Another major hurdle is the blood-brain barrier, which prevents many potential drugs from reaching affected brain regions,” said Professor Chan. As a protective shield for the brain, the blood-brain barrier blocks chemicals, harmful drugs and most bacteria – as well as therapeutic medicines.
While we don’t know what precisely causes Parkinson’s, it seems to be associated with the build-up of a protein called α-synuclein, which is involved in the release of neurotransmitters. It can come with various shapes and structures, some of which appear to lead to Parkinson’s and other neurodegenerative conditions, with disordered α-synuclein aggregating in deposits known as Lewy bodies. Unfortunately, because of its disordered structure and uncertainty in the pathway that leads to its aggregation, it has been difficult to develop therapies to target α-synuclein.
In their previous work, the team discovered a peptide derived from SUMO1 – which is similar to ubiquitin, a small protein found in the cells of most living things that helps to regulate protein degradation. This peptide has a very special quality: it binds to α-synuclein, stopping it from aggregating. It also has the handy quality of being derived from human cells, meaning that it’s less likely to be rejected by the body’s immune system.
Breaking through the blood-brain barrier
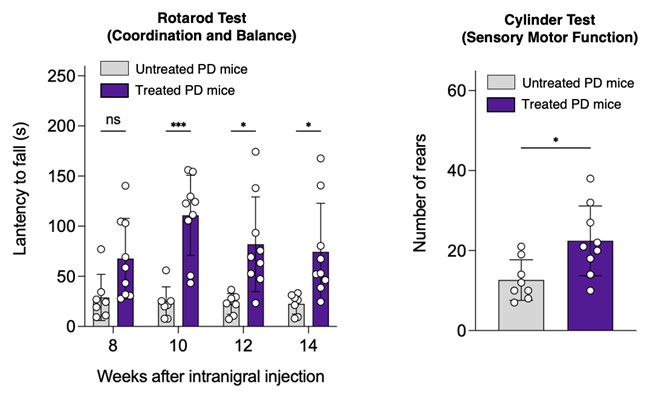
The peptide is injected directly into the brain and bypass the blood-brain barrier with the aid of a so-called recombinant adeno-associated virus, or rAAV for short, a type of virus with the viral DNA removed, commonly used as a vehicle for delivering genetic therapies within the body. When it was injected into mice, it stopped α-synuclein aggregation and protected neurons within their brains, with a demonstrable positive impact on physical Parkinson’s symptoms: for example, the mice performed better on a so-called rotarod test, meaning they were able to balance on a rotating rod for longer.
The area of the brain targeted in tests is known as the substantia nigra, which plays a vital role in motor control. “By precisely targeting this area, we aim to enhance the peptide’s neuroprotective effects against α-synuclein aggregation and neuronal loss while minimising off-target effects, improving therapeutic safety,” says Professor Chan.
“SUMO1 has been shown to enhance protein solubility and prevent aggregation when fused to target proteins. We originally planned to fuse SUMO1 to α-synuclein using a site-specific technology we had developed previously, but surprisingly discovered that SUMO1 could directly prevent α-synuclein aggregation, even without fusion.”
Handily, it appears that therapies delivered using rAAV can have an effect for upwards of a decade; a primate study of Parkinson’s suggested that they could continue to work for up to 15 years. This reduces the number of times patients need to be injected, lowering both the risk and the cost.
Towards real-world impact
To bring their research closer to clinical application, the team have set up a company, SUMO Therapeutics, in January 2025. In addition to tackling Parkinson’s, those therapies could also have an impact on Lewy body dementia, another form of degenerative disease caused by a similar abnormal build-up of proteins.
“We have proven that our peptides are efficacious in improving the motor deficits and protecting neurons from degeneration in mice,” says Professor Chan. “That’s very encouraging, and it is a major step forward towards having them tested in human clinical trials.”
Up next, they are optimising the rAAV for improved efficacy in humans and working to demonstrate the peptide’s effectiveness in other animals such as monkeys. While they are still some years away from potential commercialisation, the team are now seeking extra support from academic and industrial sources to fund their studies, as they try to arm themselves with even more effective weapons in their battle against Parkinson’s progression.