Photodynamic therapy (PDT) is a fantastic treatment for cancer. It cleverly deploys the power light, which is used to activate what’s known as a photosensitiser drug. This helps to create a class of toxic molecules called reactive oxygen species (ROS) that can target cancer cells, destroying them – and helping sick people get better.
It’s extremely effective, offering the hope of recovery to people suffering from a range of different cancers – but, crucially, not all of them. In particular, it doesn’t work effectively against solid tumours, such as those characteristic of breast cancer. That’s because it requires oxygen to work – and because of the lack of enough blood inside them due to the outgrowth of the tumours, solid tumours don’t have enough oxygen flowing through them. And that’s not the only problem: like many cancer treatments, PDT can be frustratingly scattershot, destroying not only the cancerous tissues but also the perfectly healthy tissues around them.
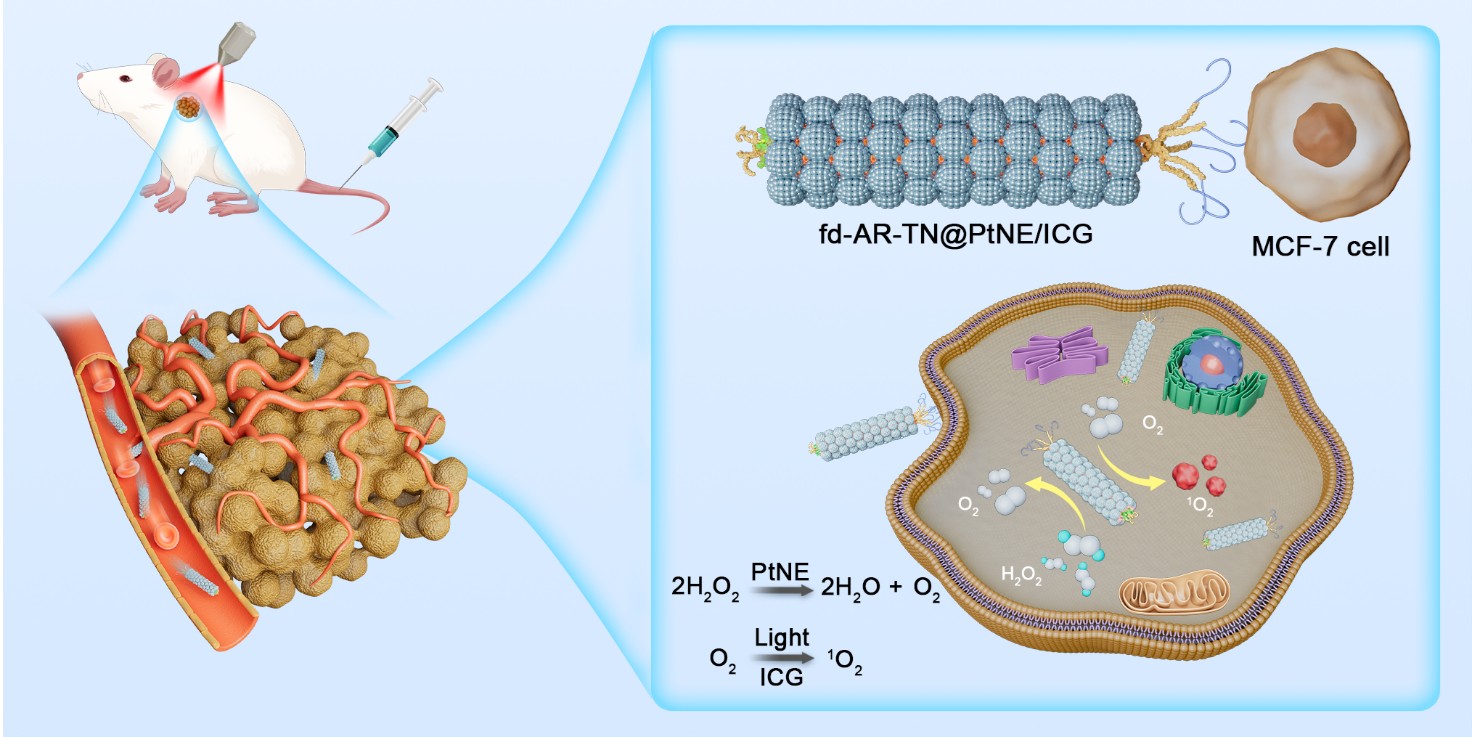
This is where a remarkable new development from a CUHK team led by the Department of Biomedical Engineering’s Professor Mao Chuanbin comes in. His new technology goes by the rather intimidating name of Tumor-Homing Phage Nanofibers for Nanozyme-Enhanced Targeted Breast Cancer Therapy, but what it does is pretty straightforward: it harnesses a type of bacteria-infecting virus to solve the problem of insufficient oxygen and zap tumours effectively.

The new technology takes a virus known as a filamentous fd phage and uses it as a biological scaffold, rebuilding it using sophisticated nanotechnology to create a therapy that mimics the structure and function of the virus.
“The filamentous fd phage virus invades bacteria,” says Professor Mao. “It is harmless to the human host. It will inject its DNA into bacteria, leading to the replication of the phage by bacteria. This is the way how we produce the phage in the lab.”
“My group has been using the phage to treat diseases such as cancer for more than a decade, so we are the leading group in phage-based therapy. The phage has also been used in other medical techniques such as treating bacterial infections because some phages can kill bacteria.”
When building the new virus-like nanofibres, Professor Mao and his team incorporated a number of extremely smart features. They are precision guided towards breast cancer cells within the body by a type of peptides known as AR, which appear at one end of them and ensure that they don’t also target health tissue (the team discovered the peptide in tests but aren’t precisely sure how it works, although they suspect it can recognise some of the receptors of the cancer cells). It proved highly effective in such precise targeting when tested on mice with a type of slow-growing breast cancer tumour called MCF-7.
Secondly, they also created their own type of artificial enzymes, known as platinum nanozymes, or PtNEs. As the name suggests, these minuscule are made enzyme-like structures are made from platinum – a metal more commonly associated with expensive jewellery. They are the magic bullet that help the nanofibres get around the whole oxygen problem. Appearing on the virus’s surface, they effectively make their own oxygen, a substance insufficient in solid tumours, by converting it from hydrogen peroxide – yes, the stuff used to dye hair, and also as an antiseptic – which is found in abundance in those tumours. When tested, the platinum nanozymes were highly effective in increasing the oxygen levels within tumours.
“When platinum forms nanoparticles, the nanoparticles can serve as a catalyst to speed up the reaction: the conversion of hydrogen peroxide into oxygen. Here, the catalytic function of the platinum nanoparticles is just like an enzyme,” says Professor Mao.
And thirdly, the team attached a light-sensitive drug called Indocyanine Green, or ICG, to the virus. Not only does this produce ROS – cancer cells’ worst enemy – when they’re exposed to near-infrared light; but, as an added bonus, it’s fluorescent, so doctors can track how the therapy is working in real time. In tests, it works really well: tumours shrank and in many cases disappeared; 40% of mice tested were cancer-free after 16 days of treatment.
“In the animal model, the cancer-killing is very efficient, as shown by the shrinking of tumours over time following the PDT,” says Professor Mao. “In control experiments, the tumours grow bigger and bigger over time.”
It also looks like the therapy could be extremely safe to use. Like the virus they’re based on, the nanofibres don’t harm people, because the fd phage only infects bacteria. When the researchers tested it in mice, it caused no damage to major organs or blood toxicity.
And there’s even better news: not only has the technology shown proven effects when it comes to treating breast cancer, but it might also have the potential to treat a range of other types of tumour, in particular melanoma. The research team are currently gearing up for human clinical trials, meaning that a lifeline for people suffering from solid tumours could be on the way soon.