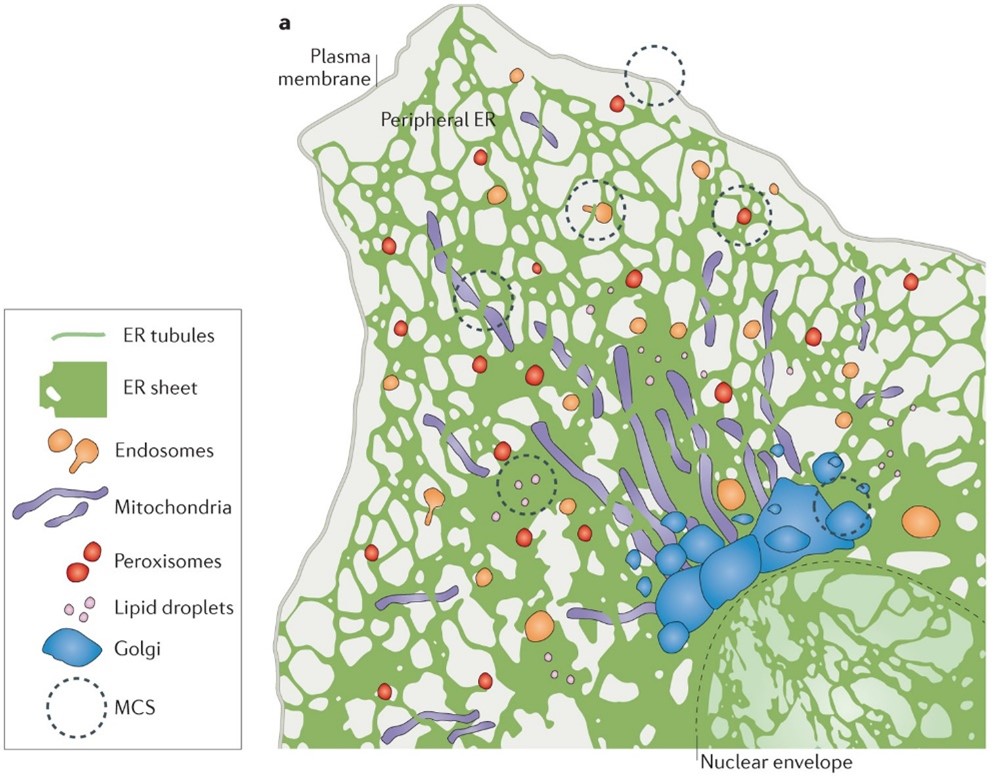
We all contain an awful lot of endoplasmic reticulum (ER). This lattice-like network is a sizeable component of nearly all of our bodies’ cells – one that’s involved in all sorts of vitally important functions. The ER is an organelle, a specialised subunit within a cell, that forms an interconnected network extending from the plasma membrane to the cell’s nucleus. Its smooth functioning is crucial for life. For years, scientists have suspected that it could be a key candidate for understanding the way those cells sense forces – the ability that allows us to live in the world, from hearing sounds to touching objects.
However, until recently, they’ve been unable to test whether their hunch is true: if the ER really does help us to sense forces around us. “The major obstacle that held back researchers from confirming the mechanosensitivity of the ER was the absence of a practical tool that allowed them to send mechanical signals directly and specifically to the organelle inside living cells,” says Professor Duan Liting, Associate Professor in the Department of Biomedical Engineering at The Chinese University of Hong Kong (CUHK).
“It is not surprising that the tools to apply force towards ER have been long unavailable, considering that ER networks spread deep inside the cells and physically connect with diverse cellular components. It is impossible for all the current methods to precisely apply forces to ER without affecting other intracellular structures. Without the right tool, it was difficult to determine if reactions of the ER to external forces were results of direct sensing or of other structures being disturbed,” says Professor Duan.
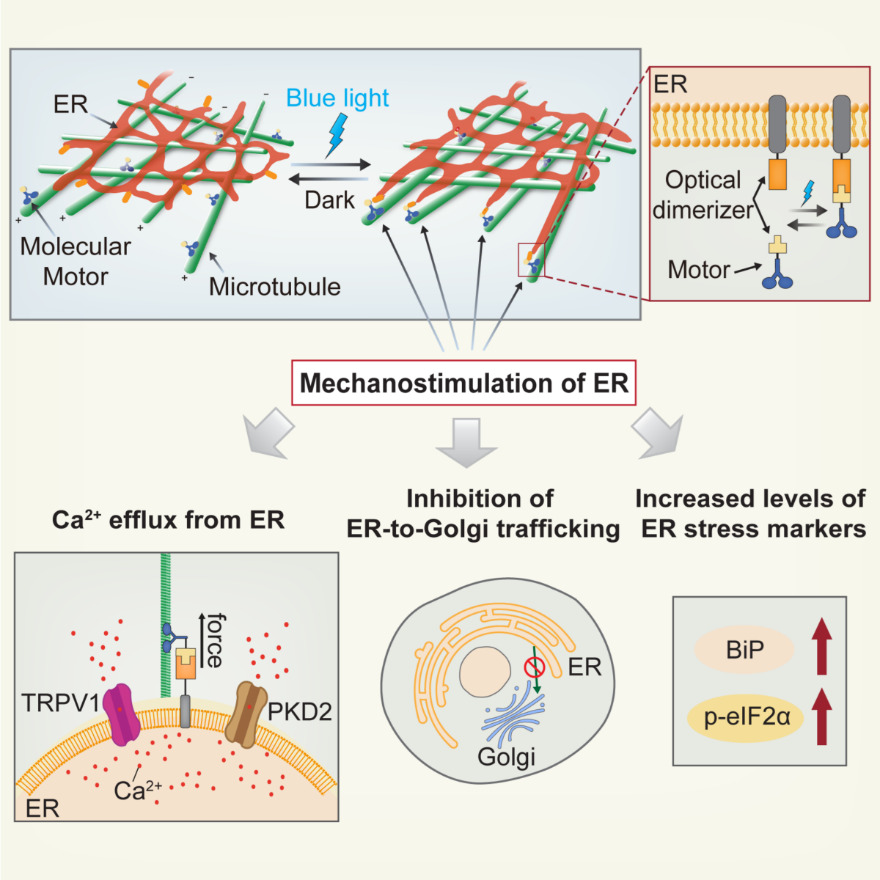
That tool has now been developed. It’s called Light-Inducible Mechanostimulator (LIMER). Created by a CUHK research team led by Professor Duan, LIMER uses light to stimulate the ER; pretty much any blue light will do it, such as from a standard blue light LED, at a wavelength of 450nm to 490nm, with an intensity of 1mW/cm2, for 100 milliseconds. That’s a very modest quantity of light – about 10% of the intensity of the blue light portion of sunlight. The light activates motor proteins within the cell, directing them to the ER, without having any impact on the other parts of the cell, by engineering the so-called force molecules, or molecular motors, to be responsive to light.
The study found that when the light is shone on it, the ER releases calcium ions – within a matter of seconds. This is important, because it shows that the ER has so-called mechanosensing and mechanoresponding abilities: it can feel external forces and respond to them. The research result has been published in Developmental Cell, the leading journal of cell biology.

Moreover, those calcium ions have all sorts of important functions within the cell. Calcium disregulation is implicated in many neurodegenerative diseases, including Parkinson’s disease and Alzheimer’s disease. “Calcium is a key signalling element in cell survival and death, and regulates almost all physiological activities,” says Professor Duan.
Understanding the role of the ER, in particular the way it regulates the release of the calcium ions, could open up the way to develop targeted treatments for various diseases. By investigating the mechanobiology of ER and how mechanical signals regulate calcium homeostasis and other ER functions using LIMER, we can better understand how changes in extracellular physical environments are connected to the pathogenesis of such diseases.
“Further investigation of the mechanobiology of the ER and how it plays a role in the pathogenesis of related diseases is definitely one of our major research interests in the future,” she adds.