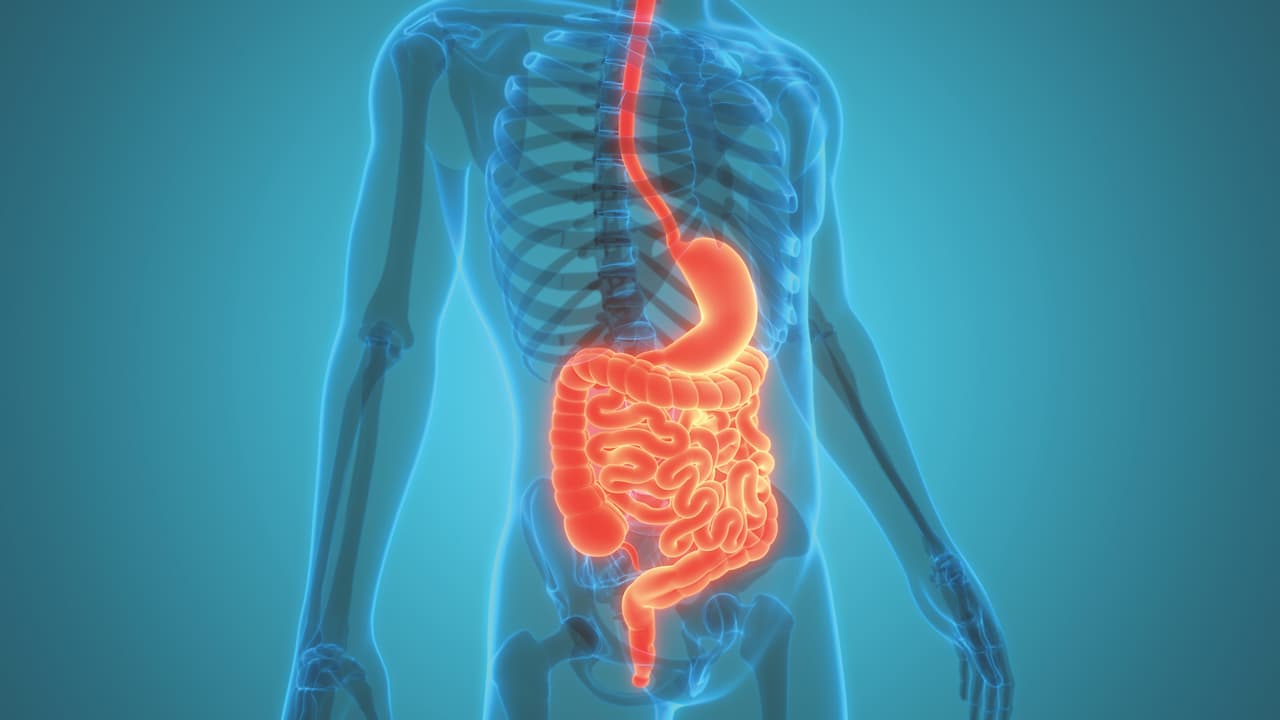
Cancers of the stomach, colon and liver are among the top causes of death globally. CUHK’s world-leading multi-disciplinary research in gastroenterology and hepatology has helped save countless lives by revolutionising early detection of gastrointestinal cancers, fatty liver disease and liver cancer. Breakthroughs in this field by Professor Jun Yu, Director of both the Institute of Digestive Disease and the State Key Laboratory of Digestive Disease at CUHK, have already been translated into clinical applications across China and Southeast Asia.
From clinic to lab
Professor Jun Yu joined CUHK in 1999 as a post-doctoral research fellow of Professor Joseph Sung Jao-yiu, the renowned researcher in gastroenterology and hepatology who later became CUHK’s Vice-Chancellor, after practicing medicine at Peking University’s Hospital.
“When I was working as a gastrointestinal specialist in Beijing, patients coming to see me were all in the late stages of cancer for which there was really no cure,” Professor Yu recalls. “As a young doctor, I felt so helpless. I wondered: Why can’t we detect these cancers early so that timely intervention is possible?” Colonoscopy is one of the most sensitive tests available for colon cancer screening and investigation. “However, because it is invasive, costly and labour intensive, it is hard to apply it for screening with the large population of China,” she says.
“I realised that the only way to make a different kind of clinical breakthrough was to do basic research to find out the cause of cancer.” She began studying the molecular mechanism that causes gastrointestinal cancers, as well as molecular alterations.

Very “basic” research
At the time Professor Yu started research at CUHK, there was no molecular non-invasive diagnostic test for colorectal cancer. A key discovery by Professor Yu and her team was that the microRNA-92 molecule present in stool is a reliable molecular biomarker for early diagnosis of colorectal cancer based on genomic and metagenomic analysis. Their fecal miR-92a diagnostic kit is the first molecular biomarker approved by the China Food and Drug Administration (now the National Medical Products Administration, or NMPA) for colorectal cancer early diagnosis in 2018.
From initial research to producing a test kit approved for nationwide application took over 15 years. At the start, Professor Yu and her students found themselves conducting the most “basic” of basic research — probing large piles of stool collected from patients to identify malignant cells shed from the thin outer layers of tissue lining their gut. “We washed the stool, removed food residues and bacteria, purified it with solution buffers, collected the cells that come off the surface of the colon, stained them with chemicals on slides and examined the cells under microscope to find any malignant cells. That phase of our research was, frankly, unpleasant. Identifying malignment cells with the naked eye under microscopy was labour intensive and ultimately not sensitive enough. So, we had to find another way.”
The CUHK team moved on to experiments with other possible biomarkers and found that a single-stranded, small non-coding RNA molecule — miR-92a — was the most stable, sensitive and cost-effective. Moreover, each test requires only 300 mg of stool sample per individual. Patent applications have been made to authorities in the US, Europe and Taiwan.
As the gut microbiome emerges as a new frontier in medicine, Professor Yu, teaming with other CUHK gastroenterologists, has developed the world’s first faecal bacterial gene marker test for detecting colorectal cancer. It is now available commercially in private hospitals and clinics in Hong Kong.
State approval
“These diagnostic kits, based on simple, low-cost tests on a PCR platform, should remain highly sustainable.”
— Professor Jun Yu
Another non-invasive blood diagnostic kit developed by the CUHK team is based on a plasma (RNF180 methylation DNA) biomarker for gastric cancer screening and early diagnosis. With a superior performance to that of conventional blood protein detection methods, it was approved by the NMPA in 2020. It is the only NMPA-approved molecular detection kit available in mainland China for the early diagnosis of gastric cancer and is commonly applied in clinical practice.
Given the rising incidence of cancer in the world, there will be increasing demand for improved diagnostic procedures to identify high-risk individuals. Professor Yu believes that these diagnostic kits, based on simple, low-cost tests on a polymerase chain reaction (PCR) platform, should remain highly sustainable.
Detecting fatty liver disease
Another research focus of Professor Yu is non-alcoholic fatty liver disease, the most common chronic liver disease globally. “Some Chinese people do not look obese. But if you check with ultrasound, their internal organs are full of fat. This is due to their rich westernised diet, which is low in fibre, and to a lack of exercise,” Professor Yu says.
“Non-alcoholic steatohepatitis (NASH) is the more severe form of fatty liver disease and can progress silently towards cirrhosis and liver cancer,” Professor Yu cautions. “Liver biopsy remains the gold standard for its diagnosis, but it is limited in routine clinical practice due to its invasiveness and high cost, among other reasons. Many Chinese will not accept having a piece of their liver sliced,” she says. “That’s why we set out to find another way for early detection.”
Recently, Professor Yu and her team discovered a group of blood-based biomarkers for diagnosis of NASH. She has applied for a patent for the technology on the mainland. This research on non-alcoholic fatty liver and its related cancer has, for the second time, earned Professor Yu and her CUHK colleagues the prestigious State Natural Science Award (second-class).